39 fda requirements food labels
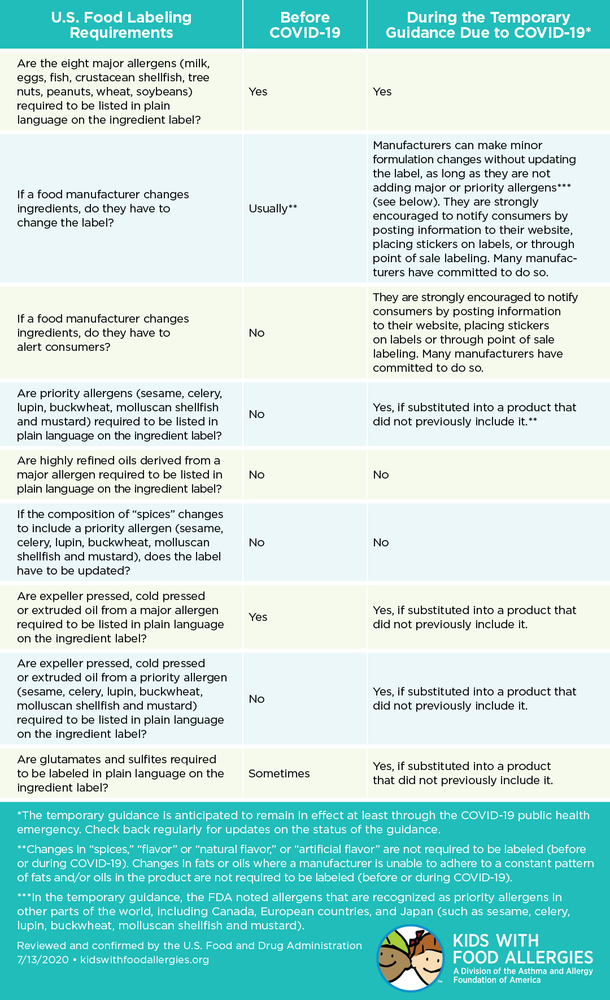
USA Food Labeling Regulations - FDABasics Animal (Pet) Food Labeling Requirements in the USA Animal or Pet food products sold in the USA must contain the below-listed information. Product identity Guaranteed analysis Name and place of the business Calorie Statement Feeding Direction Nutrition Adequacy Statement The above-listed requirements briefly summarize the labeling requirements. Food Labeling Requirements for FDA Compliant Label Design - enKo Products The FDA requires the following business details on food labels: Business name Street address City or town State Zip Code Indicate if the business name is that of the manufacturer, distributor, importer, etc. If the product is exported or manufactured outside of the US, the country of origin must appear conspicuously on the label for food safety.
Food Labeling - USDA Several federal agencies are involved in the regulation of food labels in the United States. Food labeling is generally regulated by the United States Department of Agriculture (USDA) and the United States Food and Drug Administration (FDA). The Food Safety and Inspection Service (FSIS), a public health agency within the USDA, is responsible for ensuring that the nation's commercial supply ...
Fda requirements food labels
FDA Food Label Compliance - Label Review Fees - fdahelp.us The Food Manufacturer or Distributor is responsible for ensuring their food product labeling complies with FDA regulations. FDA will not review or approve food labels. If the labels do not comply with FDA requirements, the FDA will consider the product as misbranded and may take regulatory action, including detention. LMG's Label review service ... Packaging and labelling | Food Standards Agency Food businesses must include a business name and address on the packaging or food label. This must be either: the name of the business whose name the food is marketed under; or the address of the... FDA Label Search - Food and Drug Administration Unapproved Drugs: Drugs Marketed in the United States that Do Not Have Required FDA Approval, where information about unapproved animal drugs products is available. Downloadable SPL data; Send questions and comments to the SPL Coordinator at spl@fda.hhs.gov. Food and Drug Administration - -
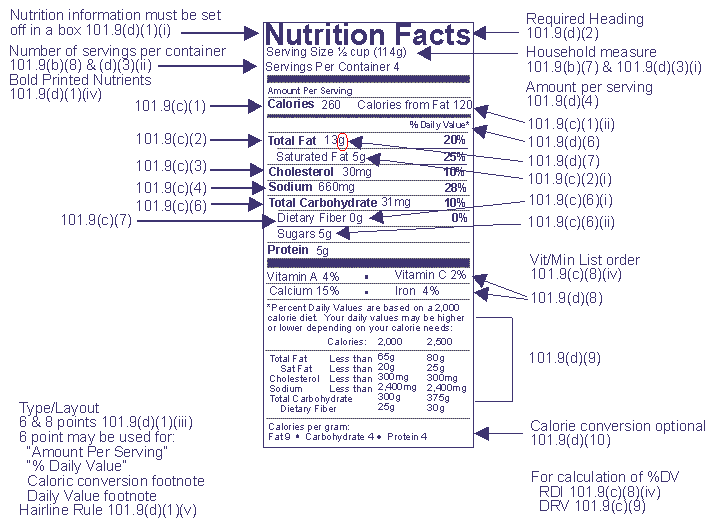
Fda requirements food labels. US Food Labeling Requirements: How Businesses Can Comply When labeling a product, there are up to eight specific labeling requirements. Four of the requirements are required to be on the Principal Display Panel. Those requirements include the product name, handling statement, inspection legend/establishment number, and net weight. The other four required fields can be placed on the Principal Display ... CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (a)(1) Ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be... Food Labeling 101 - FDA Regulations Guide [2022] | Artwork Flow Food Labeling Requirements As Stated By The FDA I. Principal Display Panel. II. Information Panel. III. Nutrient Panel. IV. Claims And Warnings. The term Principal Display Panel a.k.a. PDP refers to the front part of a food product... Food Product Labeling Requirements of the FDA The FDA Labeling Regulations define food as any processed substance, which is intended for human consumption, and includes drinks for human beings, beverages, chewing gum and any substance which have been used as an ingredient in the manufacture, preparation or treatment of food. What is a product label or labeling?
Food Product Dating | Food Safety and Inspection Service Federal regulations require a "Use-By" date on the product label of infant formula under inspection of the U.S. Food and Drug Administration (FDA). Consumption by this date ensures the formula contains not less than the quantity of each nutrient as described on the label. Guidance for Industry: Food Labeling Guide | FDA It is the responsibility for the food industry to remain current with the legal requirements for food labeling. All new regulations are published in the Federal Register (FR) prior to their... FDA Food Regulations | FDA Food Labeling Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and fish is voluntary. Under FDA's laws and regulations, FDA does not pre-approve labels for food products FDA Proposes to Update the Definition of "Healthy" on Food Labels ... On September 28, 2022, the Food and Drug Administration (FDA) announced its proposal to update its 1994 definition of "healthy" when used on food labels and labeling to align with the current nutrition science, the Dietary Guidelines for Americans, and updated Nutrition Facts label requirements.Comments on FDA's proposed rule can be submitted until December 28, 2022.
The FDA is updating the definition of 'healthy' and designing new labels It is a complex question, but the Food and Drug Administration aims to help answer it with a new food package labeling system. The last time the agency defined healthy was back in 1994. That was ... Fda packaging and labeling requirements - necq.jackland.shop Labeling Guidelines. Any pharma label printing for pharmaceutical use is required by the FDA to be designed and applied in such a way that it can remain in place, and be legible in a number of environments like use and storage for the entire lifespan of the product. All pharmaceutical labels need to display certain information on their labels. CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (k) The label of a food to which any coloring has been added shall declare the coloring in the statement of ingredients in the manner specified in paragraphs (k)(1) and (k)(2) of this section, except that colorings added to butter, cheese, and ice cream, if declared, may be declared in the manner specified in paragraph (k)(3) of this section, and colorings added to foods subject to §§ 105.62 and 105.65 of this chapter shall be declared in accordance with the requirements of those sections. eCFR :: 21 CFR Part 101 -- Food Labeling (1) Ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be listed by common or usual name in descending order of predominance by weight on either the principal display panel or the information panel in accordance with the provisions of § 101.2, except that ingredients in dietary supplements that are listed in the nutrition label in accordance with § 101.36 need ...
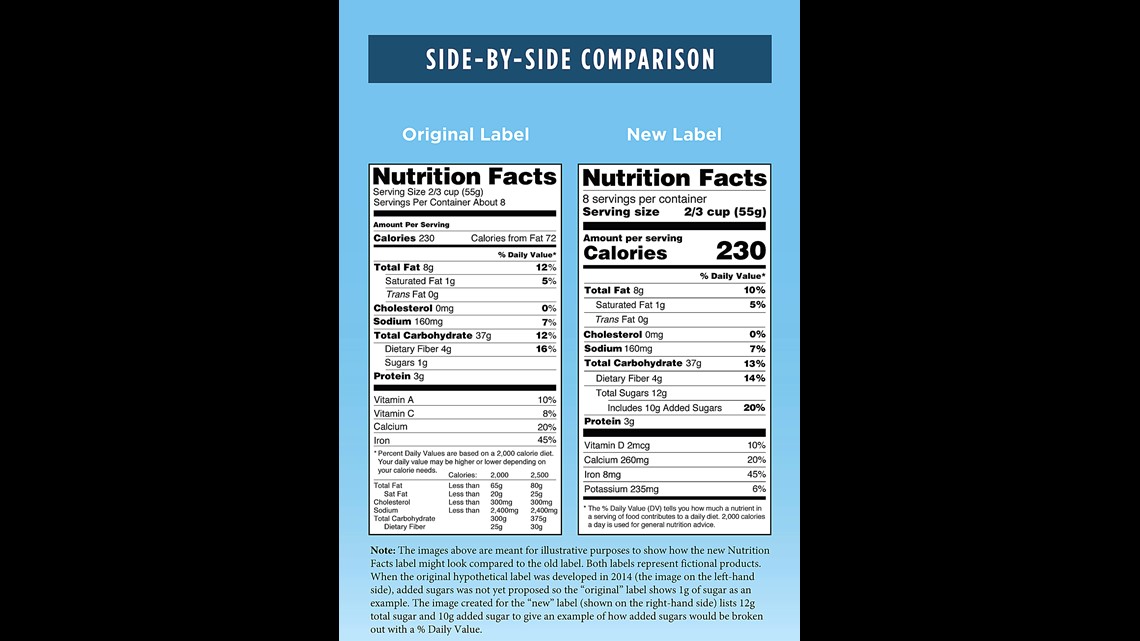
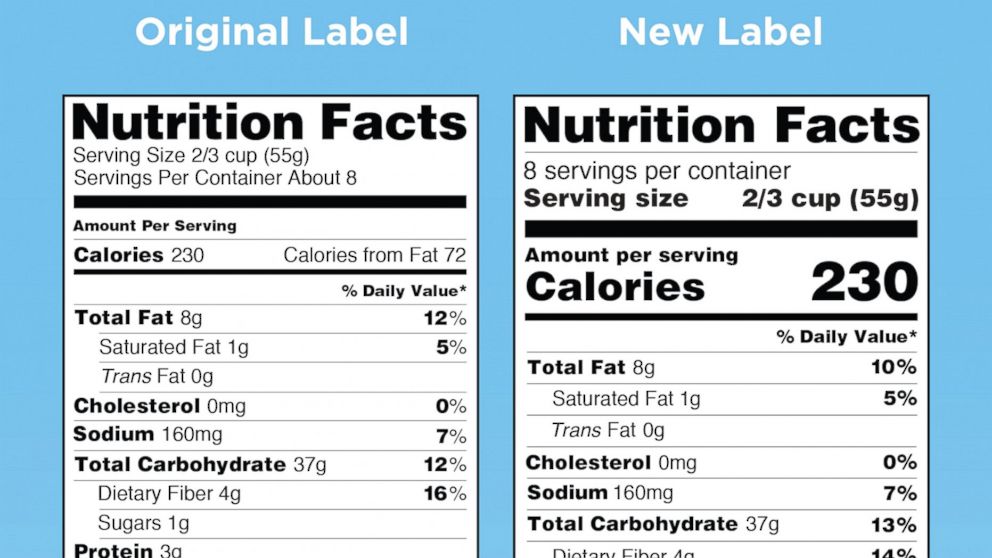
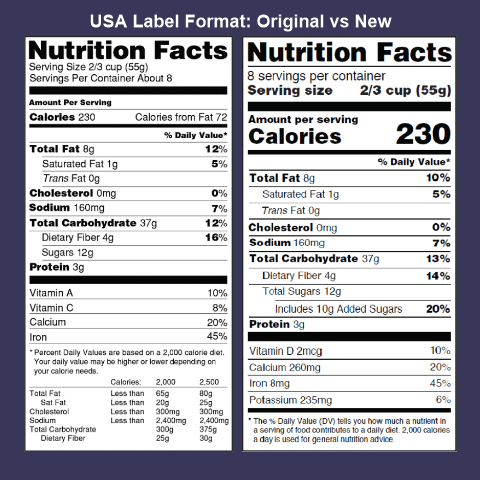
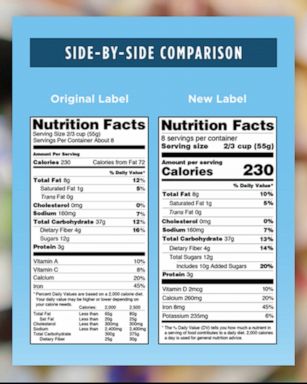
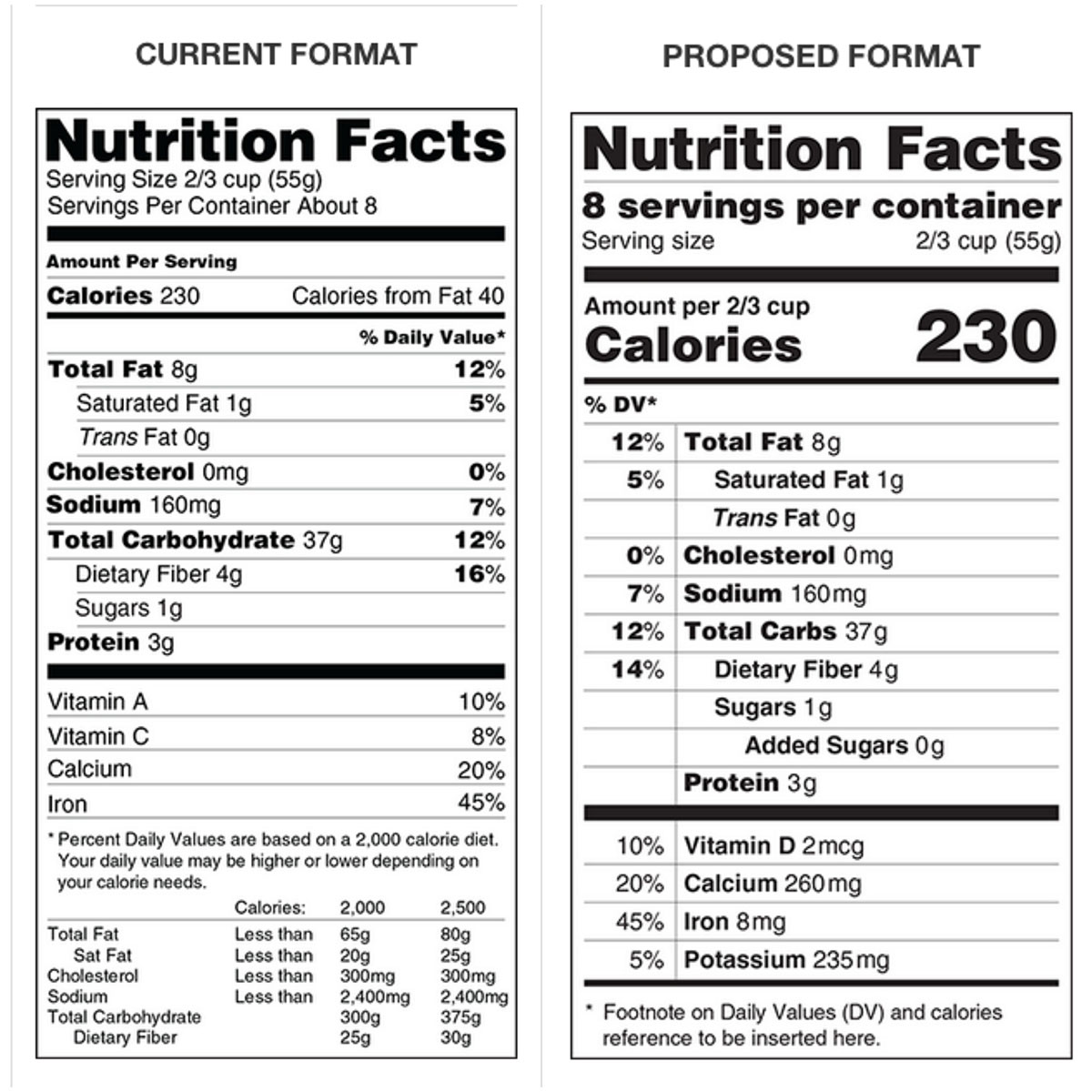
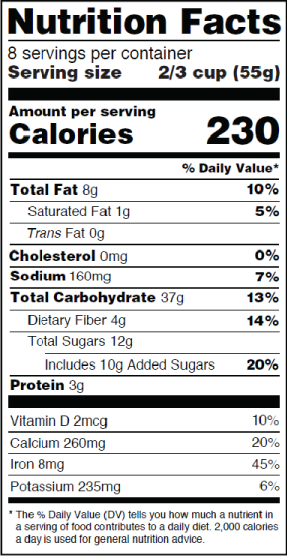
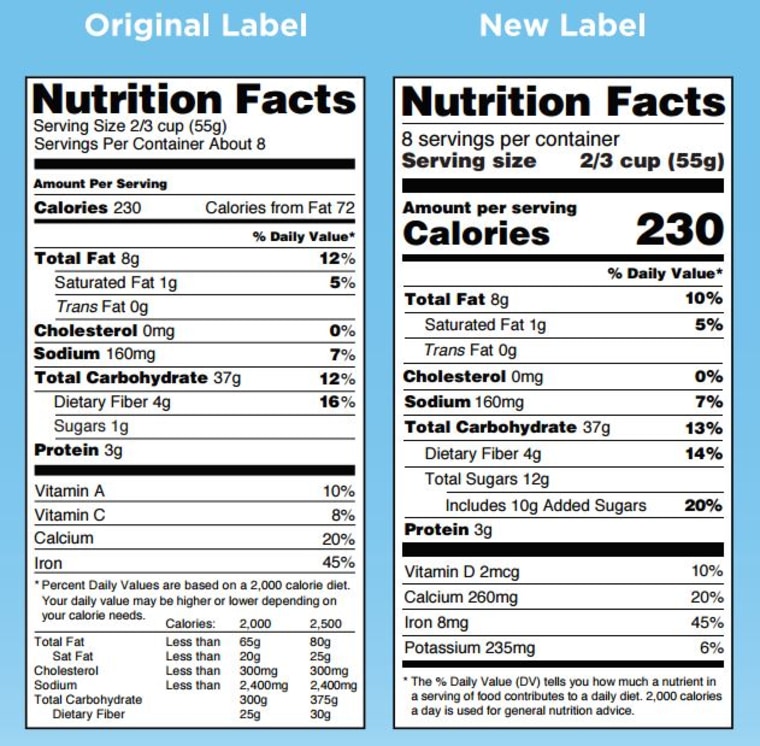
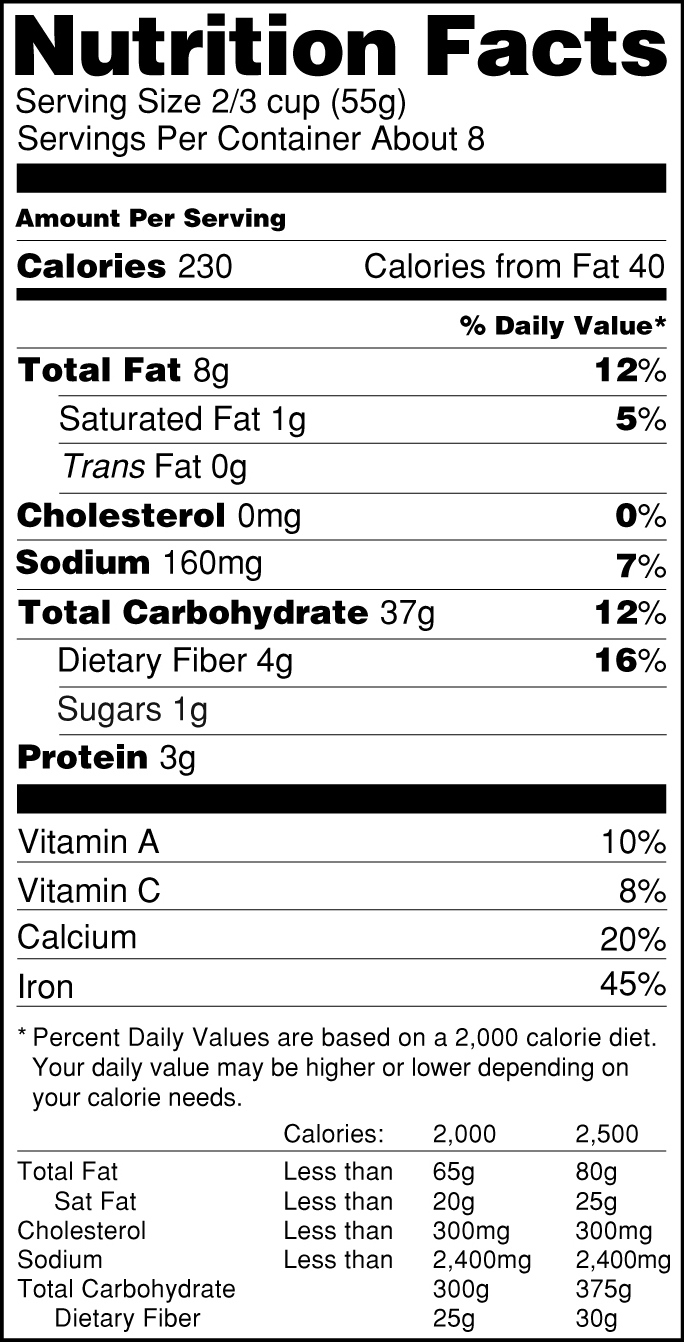
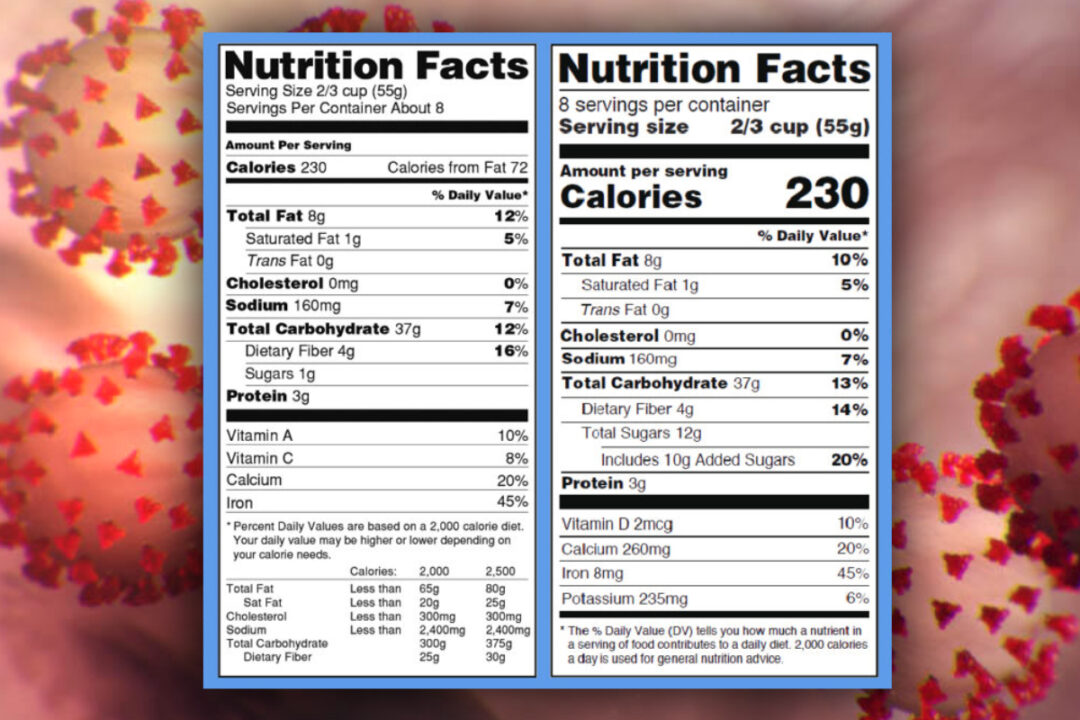
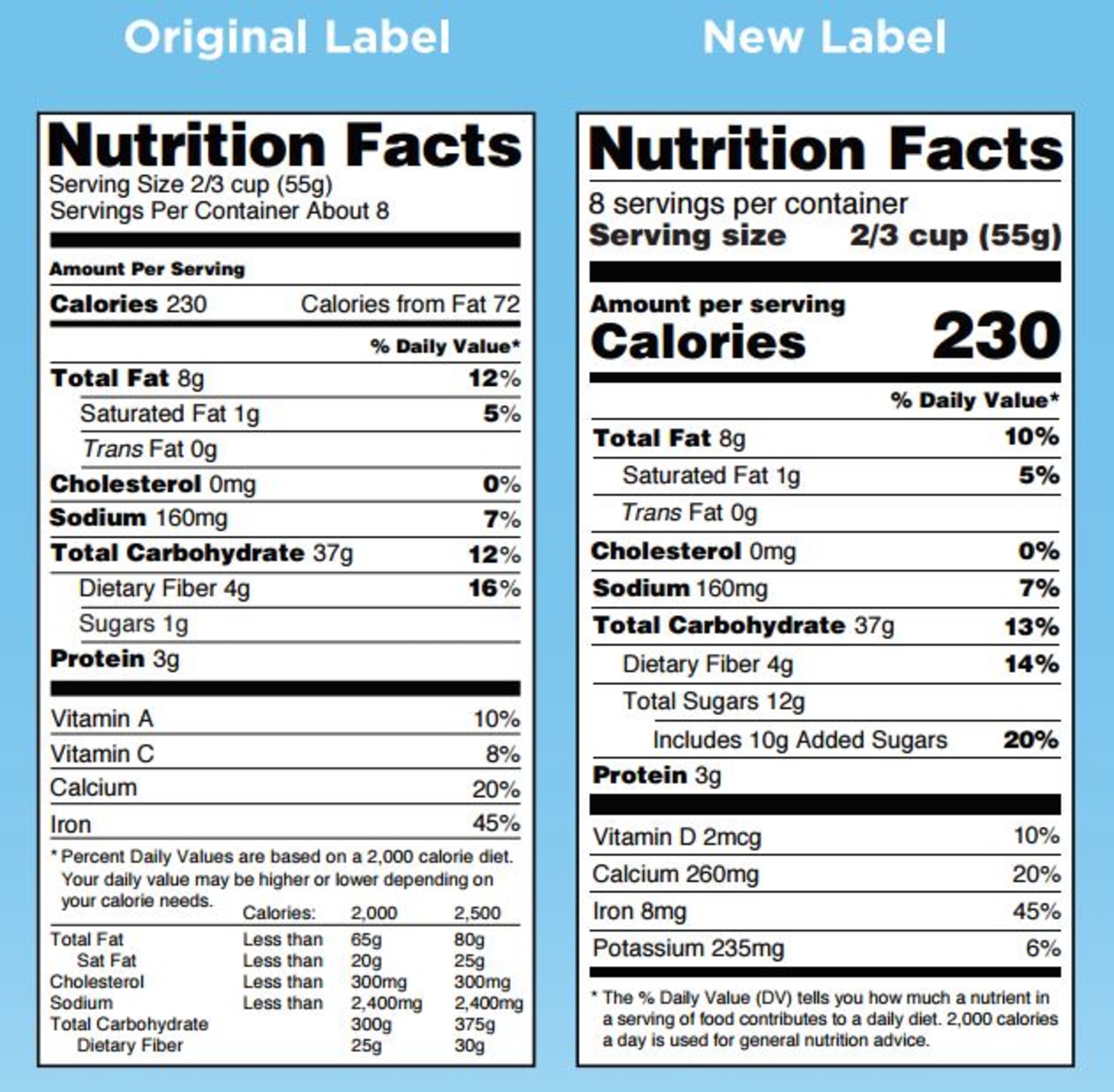
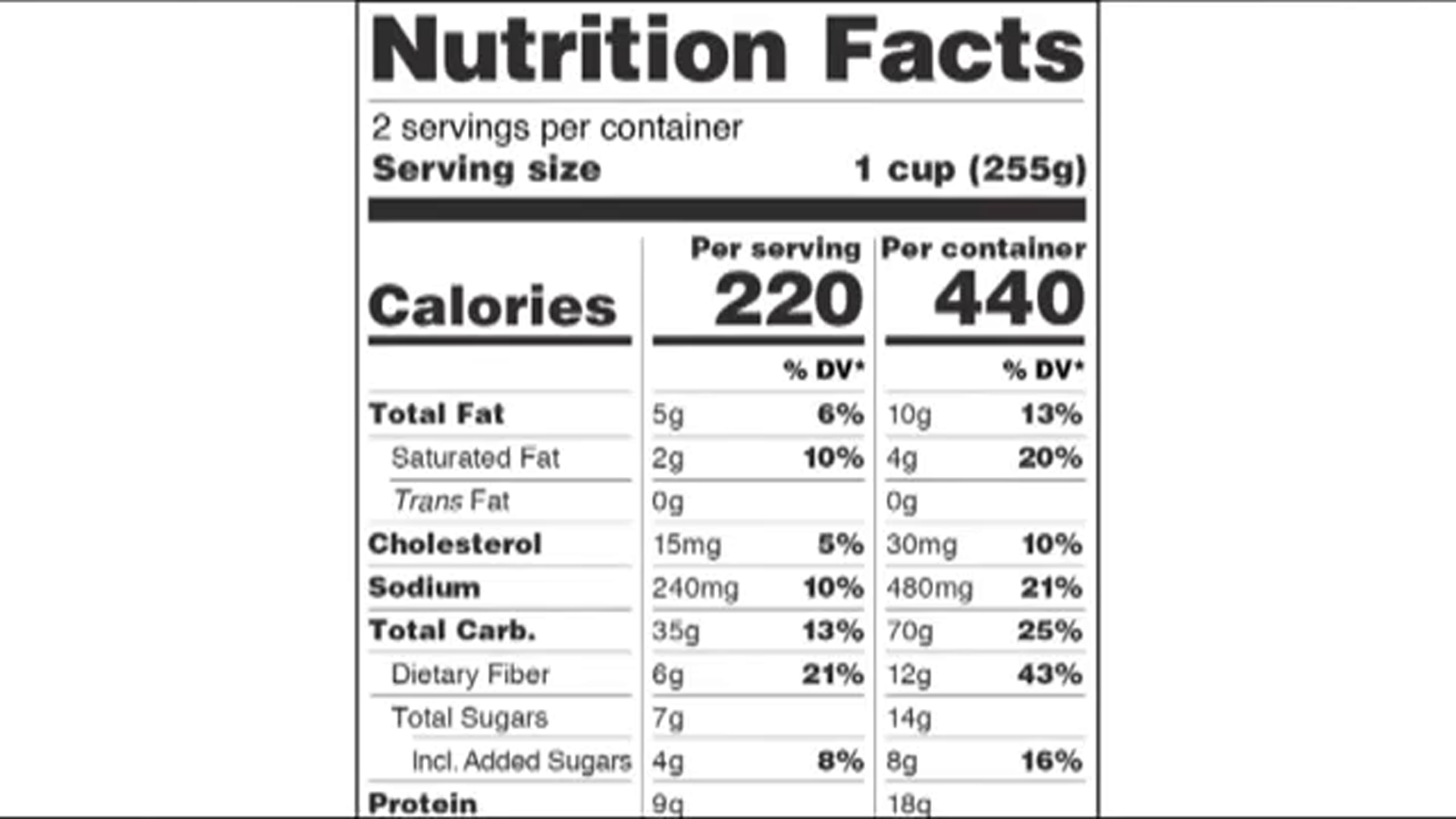
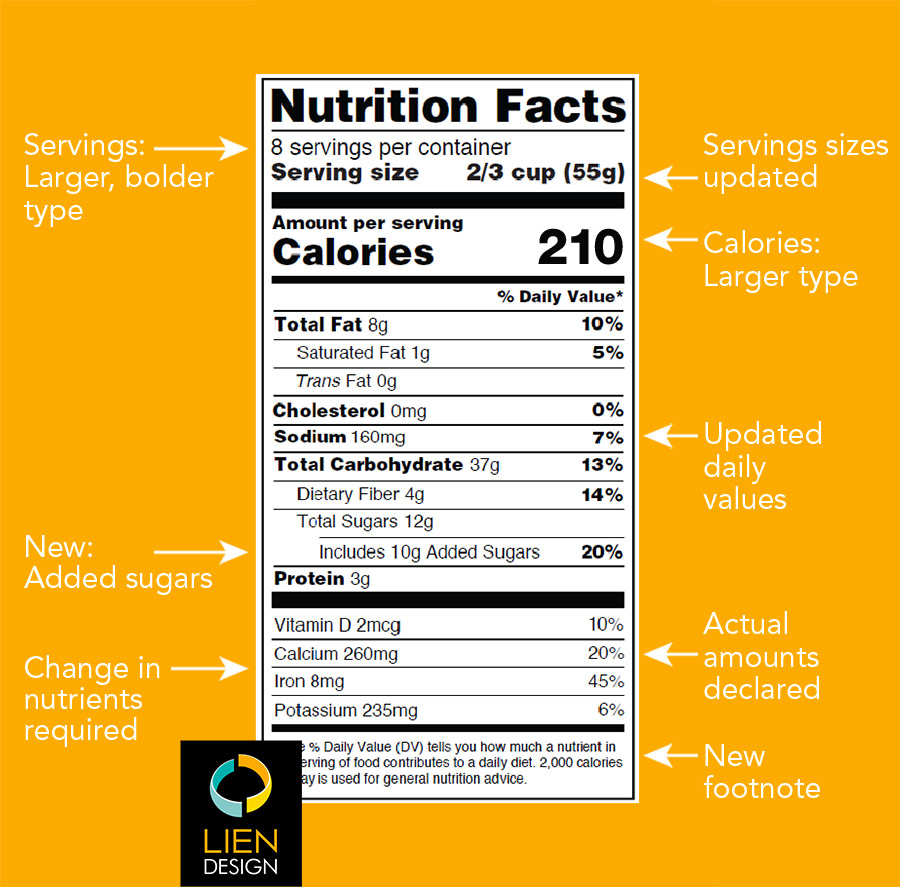
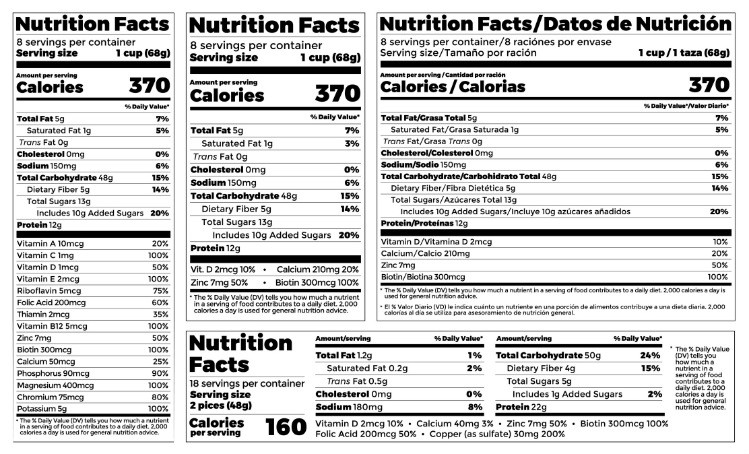
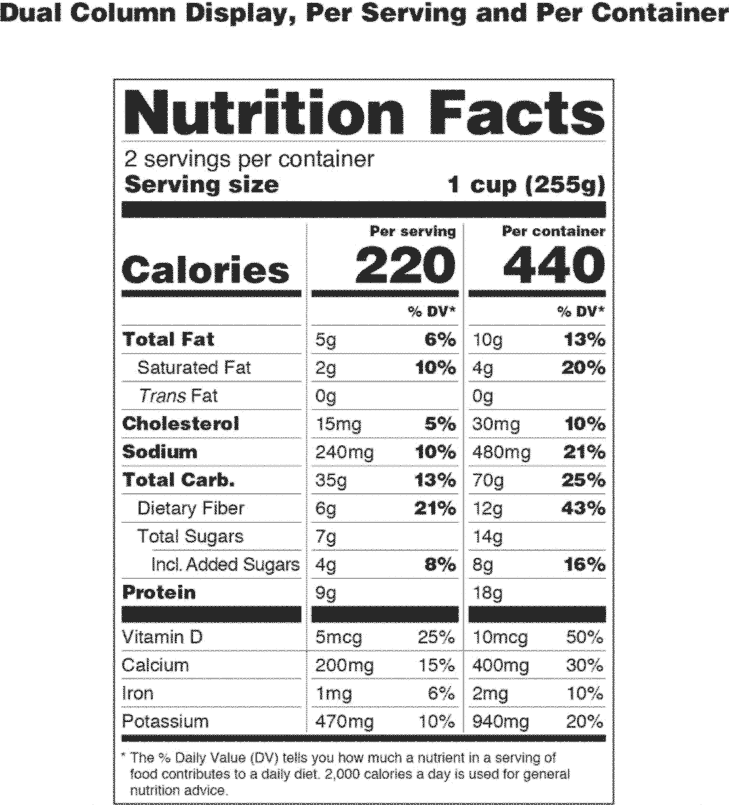
The New Nutrition Facts Label | FDA - U.S. Food and Drug Administration The U.S. Food and Drug Administration (FDA) has updated the Nutrition Facts label on packaged foods and drinks. FDA is requiring changes to the Nutrition Facts label based on updated scientific ...
What are the FDA requirements for food- USA Food regulations Labeling is one of the important FDA requirements for food products. We can offer labeling review services and our fee for per product labeling review is $ 299. In order to review the label, you should provide us label design in PDF or image format. We offer discounts on multiple labeling reviews.
2020 FDA Regulations for Food Labeling - LabelCalc 2020 FDA Regulations for Food Labeling: Are You Compliant? According to new FDA regulations regarding food labeling for food manufacturers: companies exceeding $10 million in revenue must comply with new changes by Jan 1, 2020. Companies below that revenue mark or single supply manufacturers of items such as sugar and honey have until Jan 2021.
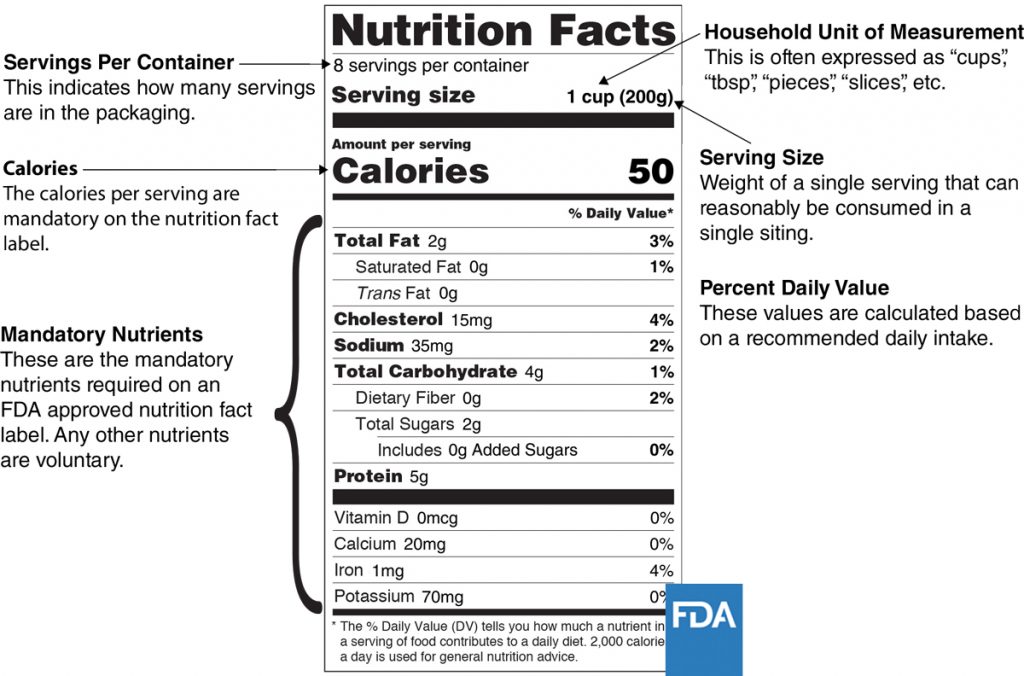
Basics of FDA Food Labeling Requirements — FDA Reader Some products are exempt from labeling requirements. Your food product must be labeled with the following labeling elements. The required location of these elements is in parentheses, when applicable. Common name of the food (Principal Display Panel) Net quantity of contents (PDP) Ingredient list (PDP or information panel)
Auburn food historian explains new FDA guidelines for 'healthy' food labels Article body. Last week, the U.S. Food and Drug Administration, or FDA, updated its criteria for foods labeled "healthy." The proposed change is based on current nutrition science and prioritizes healthy dietary patterns, continuing from the FDA's overhaul of the Nutrition Facts panel in 2016. Assistant Professor of History Xaq Frohlich explains why and how "healthy" food label ...
Food labelling - general EU rules - Your Europe Labelling. Mandatory information must be printed using a font with a minimum x-height of 1.2 millimetres. If the largest surface area of packaging is less than 80 cm², you can use a minimum x-height of 0.9 mm. For packaging surface of less than 10 cm², you must list: name of the food. any substances or products causing allergies or ...
FDA Food Packaging Guidelines for 2022 | Newprint Nutritional labels must be printed in all black or one colour type on a white or neutral contrasting background and must be readable. There have been changes in the FDA food labeling requirements regarding nutrition facts, and the transition period has ended on January 1, 2021. Health Canada closely regulates the size and appearance of the NFT.
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and...
FDA Rounding Rules for Your Food Label - LabelCalc As a food manufacturer, you know your food labels must comply with FDA guidelines for nutrition information. And while these guidelines are important in providing transparency about your products so consumers can make educated decisions, the rules can be overwhelming and complicated to navigate.
A Guide to Federal Food Labeling Requirements for Meat, Poultry, and ... A Guide to Federal Food Labeling Requirements for Meat, Poultry, and Egg Products Guideline ID FSIS-GD-2007-0001 Issue Date August 2007 Full Guideline FSIS-GD-2007-0001 This guidance document assists firms in the development of food labels that meet FSIS requirements. This guidance document relates to FSIS labeling regulations in 9 CFR 317 and 381.
FDA Food Product Labeling & Packaging Requirements - ESHA Food Product Labeling and Packaging 101. The FDA regulates most packaged foods sold in the United States and has specific requirements for what elements a package must contain (e.g. a Nutrition Facts panel, ingredient statement, etc.). In order to sell your food products, you must comply with the FDA's packaging laws unless your operation is exempt.
FDA Label Search - Food and Drug Administration Unapproved Drugs: Drugs Marketed in the United States that Do Not Have Required FDA Approval, where information about unapproved animal drugs products is available. Downloadable SPL data; Send questions and comments to the SPL Coordinator at spl@fda.hhs.gov. Food and Drug Administration - -
Packaging and labelling | Food Standards Agency Food businesses must include a business name and address on the packaging or food label. This must be either: the name of the business whose name the food is marketed under; or the address of the...
FDA Food Label Compliance - Label Review Fees - fdahelp.us The Food Manufacturer or Distributor is responsible for ensuring their food product labeling complies with FDA regulations. FDA will not review or approve food labels. If the labels do not comply with FDA requirements, the FDA will consider the product as misbranded and may take regulatory action, including detention. LMG's Label review service ...

















Post a Comment for "39 fda requirements food labels"